Overview of Our Research
Reproducing biological functions in a simple protein scaffold represents the ultimate test of our understanding. We must know the roles of each amino acid involved, as well as how the structure of the protein itself factors in. In the Solomon lab, we start with a simplified protein or protein material and meticulously make small changes to reproduce our desired function. In so doing, we learn more about the minimal protein-engineering required. Furthermore, our bottom-up approach removes much of the complexity seen in natural proteins, so we are not fighting against evolution.
This approach also lets us develop new functions that did not exist before in these scaffolds and the malleability of our system lets us port these biological functions to new contexts. In the Solomon lab we utilize two distinct model systems in which to test our knowledge of function.
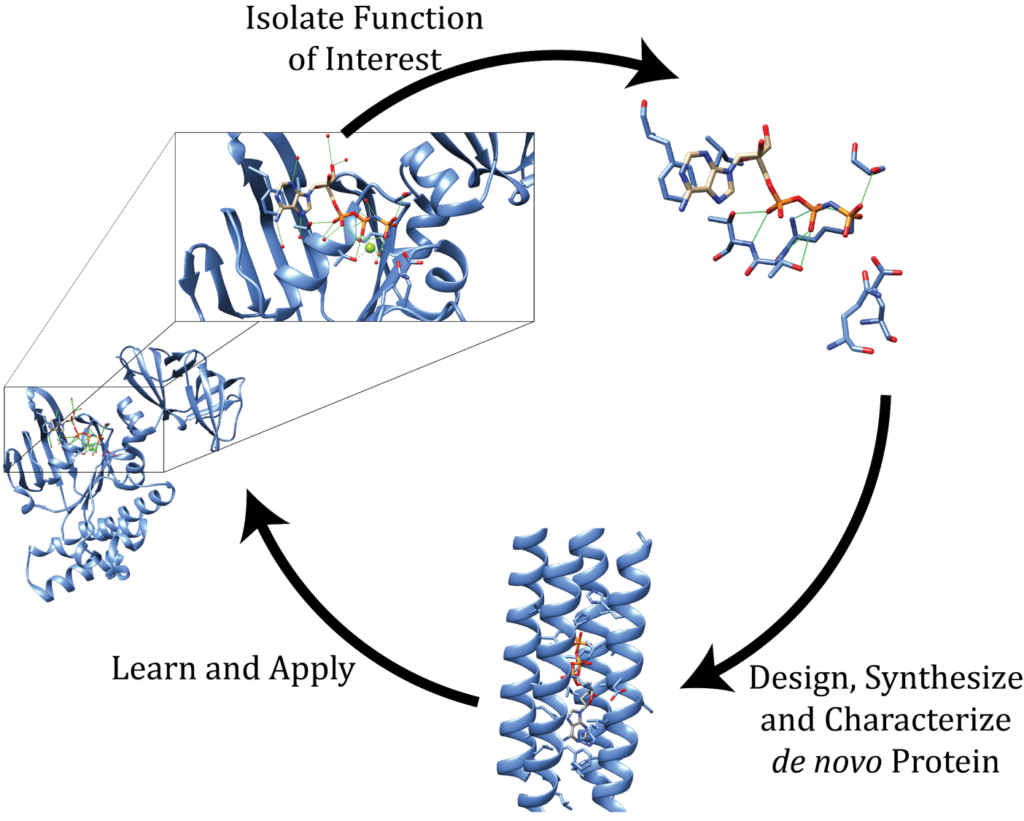
Monomeric Proteins
In the Solomon lab we are using monomeric protein maquettes. 4-alpha helical bundle proteins that have been developed over the years to carry out a myriad of impressive functions ranging from oxygen binding to carbene transfer. In the Solomon lab, we are interested in expanding their functions even further.
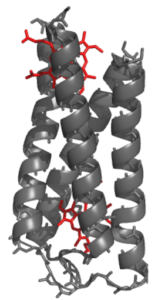
DNA Binding
 DNA Binding
DNA Binding
In the Solomon lab we are looking to use de novo proteins to tackle an important biological challenge: Can we make designer DNA-binding proteins? This phenomena is at the heart of most genetic processes and accordingly central ot many disease states and disorders. We are engineering proteins that can bind to a series of DNA sequences and then function as artificial transcription factors. We aim to apply this work toward a host of synthetic biological applications and as a new way to treat genetic disorders
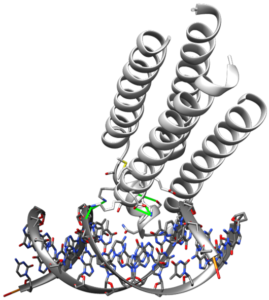
ATP Binding and Phosphoryl Transfer
 ATP Binding and Phosphoryl Transfer
ATP Binding and Phosphoryl Transfer
The Solomon group is interested in develop artificial kinases. ATP is the energy source of the body. Transferring a phosphate to another molecule is used to drive unfavorable chemical reactions as well as mediate signaling pathways and cascades. This phosphoryl transfer reaction so prominent, it is believed that up to 30% of all human proteins are phosphorylated at some point in their lifecycle. We are interested in reproducing this function in a simplified protein scaffold and creating an enzyme that can transfer phosphate to substrate of our choice. With this technology we will better understand how proteins catalyze this powerful reaction and
Peptide Amphiphile Materials
Our lab uses peptide amphiphiles to study natural functions on a larger scale. Peptide amphiphiles are short peptides connected to a lipid tail. Since their introduction their versatility and potential have lead to them being developed for a variety of functions ranging from drug delivery to the regrowth of neural tissue and regaining of sensation in paralyzed mice. In the Solomon lab we are interested in exploring their oxidoreductase properties and measuring their material properties in different environments.
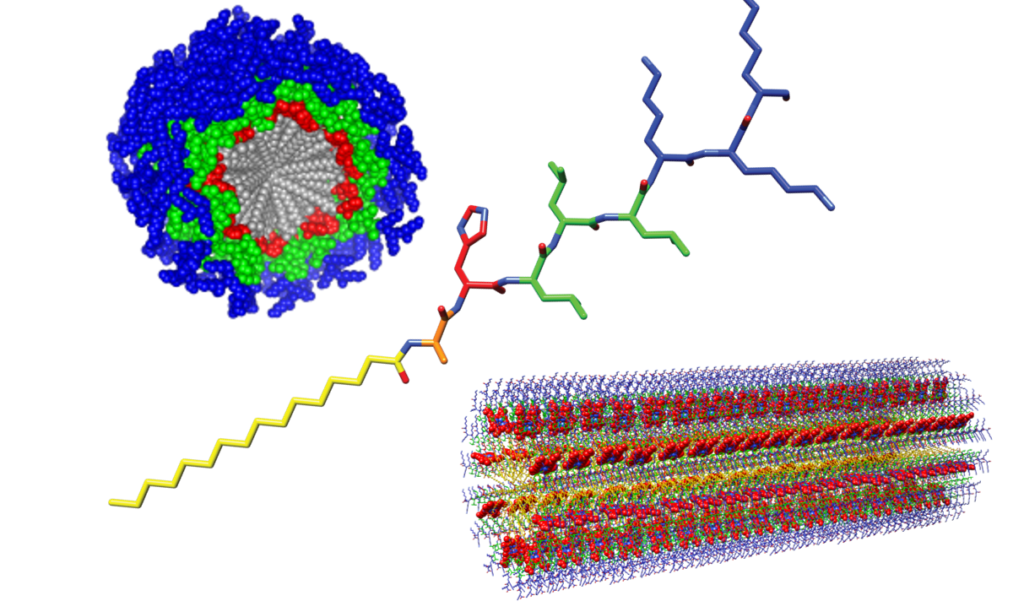
Peptide Wires
 Peptide Wires
Peptide Wires
We are using our polymeric peptide material to engineer the next generation of electrical wiring where the wire itself can take part in the information processing. The peptides we are working with bind redox cofactors which allow us to explore a variety of oxidoreductase functionalities. The simplicity of our system means that our peptide materials can also be used as a model system to study other natural proteins that carry out long range electron transport like the OmcS protein of Geobacter sulfurreducens. We can also incorporate a variety of other oxidoreductase cofactors to bring in new functions and better study their properties in a simplified peptide material
Peptide Wound Dressings and Artificial Blood
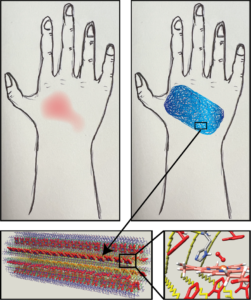 Peptide Wound Dressings and Artificial Blood
Peptide Wound Dressings and Artificial Blood
We are also using our polymeric proteins to study oxygen binding. Our peptides use the same metal cofactor found in hemoglobin and we are developing them in such a way that they reproduce this function. With that we can use this technology to develop blood substitutes to alleviate consistent supply shortages in emergency medical settings and wound dressings that can deliver oxygen abd speed up the healing process. We can also learn more about the biophysical underpinnings of natural oxygen binding proteins which we hope will lead to new medical interventions to help hypoxic patients.